Drug Regulatory Authority of Pakistan (DRAP) on Wednesday conducted a crackdown against fake imported medicines and barred patients from using or purchasing the injection – Rhophylac 300.
In its product recall alert, DRAP warned patients to avoid the use of the human anti-D immunoglobulin drug ‘Rhophylac’, as the sample label of the injection – manufactured by Swiss company CSL Behring – printed incorrect details.
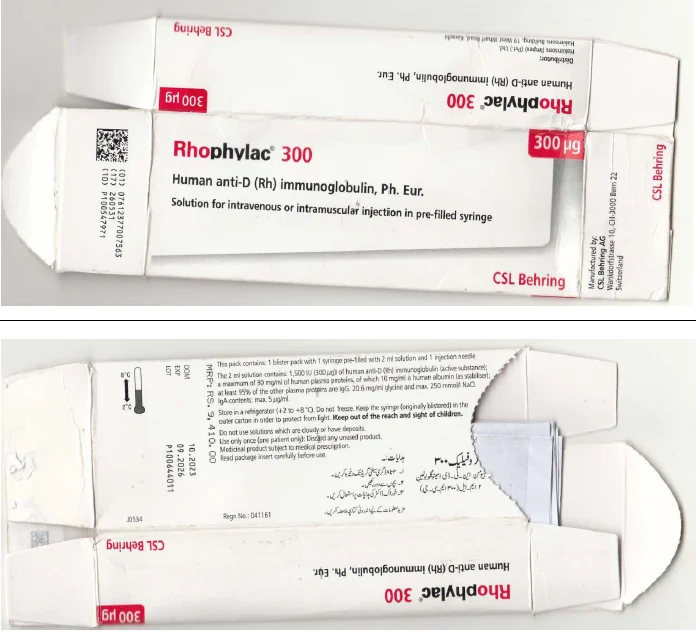
The KP Drug Inspector and a Karachi-based pharmaceutical company have reported the circulation of fake ‘Rhophylac’ injections in the market.
These injections, commonly used for treating immune thrombocytopenic purpura, were found to have discrepancies in their batch numbers and barcode scans.
The counterfeit nature of the product raises significant health concerns as its quality and effectiveness cannot be guaranteed.
The DRAP stated that the drug, derived from human blood plasma, is used to target red blood cells, and any counterfeit version could be harmful to patients’ health.
DRAP has ordered the seizure of the affected batches of Rhophylac from the market to prevent further distribution and safeguard public health.
Earlier this month, DRAP banned the sale and use of Famila injection, a contraceptive injection found to be substandard and potentially harmful for females.
After confirming that a specific batch of the contraceptive injection did not meet safety standards, the central drug testing lab flagged the product – manufactured by Zafa Pharmaceutical Karachi.
In response, DRAP has issued a product recall alert, urging the removal of the affected Famila injection batch from the market in Karachi, as reported by ARY News.
The alert underlines that using the substandard batch may pose serious health risks, including blood infections.